TEST PROTOCOLS
Computer System Validation
Computer System Validation
cGMP Consulting Inc. validates computerized systems from stand-alone instruments, with associated controls, to enterprise-level enablement. Our specialists provide an accurate assessment of computer system compliance and/or validation against regulatory and industry standards which comply with the following requirements:
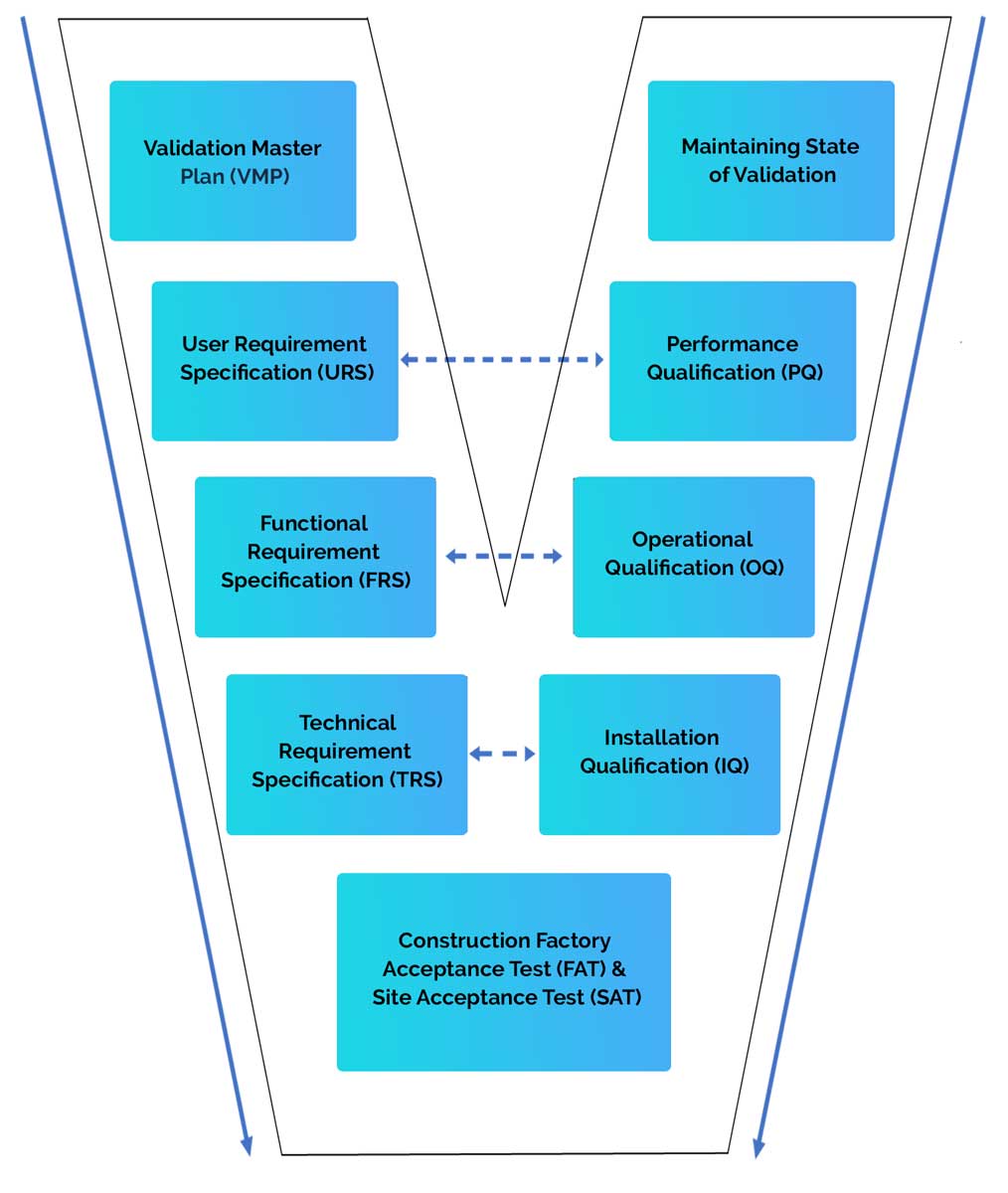
- GAMP5 software life cycle development model (V model)
- FDA 21 CFR Part 11 on electronic records, electronic signatures
- PIC/S annex 11 on validated computer systems
cGMP Consulting Inc.’s validation consultants develop and implement strategic testing protocols to meet:
- User Requirement Specification (URS)
- Functional Specification (FS)
- Design Specification (DS)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Automated Process Controller Installation and Operational Qualification (APCIOQ)
Whether it is a new computer system, or modifications to an existing one, it all starts with a User Requirements Specification (URS). A URS describes the functional and non-functional requirements of the software and hardware in order to meet the user’s needs.
cGMP consultants can help you develop a URS that forms the basis for the design and development (if any) of the computer system. They will ensure that your URS is complete, realistic, definitive and testable.
By using Quality by Design principles, cGMP consultants can perform design reviews to determine the optimal design of the computer system, ensuring quality, safety and effectiveness are designed and built into the software.
Qualification or test protocols are used to demonstrate that a system meets requirements previously established in specification, design, and configuration documents. cGMP’s validation contractors are experts in developing and implementing strategic testing protocols to meet:
- Installation Qualifications (IQ): verify that systems are on machines suited to run the software, that the system has been properly installed and that the configuration is correct. These requirements are outlined in the Design Specification.
- Operational Qualifications (OQ): verify that systems perform as expected. The OQ tests requirements outlined in the Functional Requirements.
- Performance Qualifications (PQ): verify that systems perform tasks in real-world conditions. The PQ tests requirements outlined in the User Requirement Specification.