
How Industry 5.0 is Revolutionizing cGMP: What Manufacturers Need to Know
Introduction: The New Industrial Paradigm
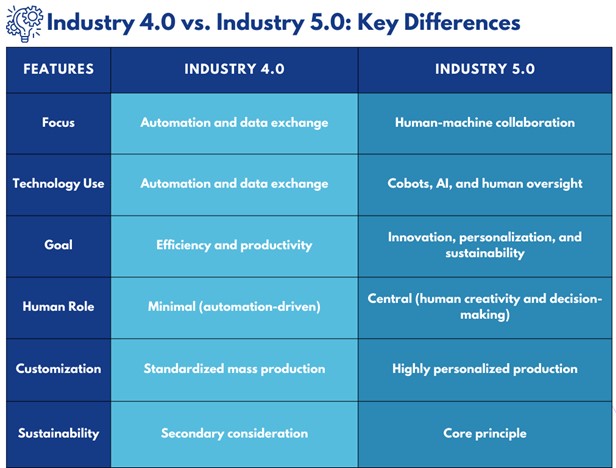
Industry 5.0 represents a paradigm shift in manufacturing, emphasizing collaboration between humans and advanced technologies like artificial intelligence (AI), robotics, and the Internet of Things (IoT). Unlike Industry 4.0, which primarily focuses on automation and data exchange, Industry 5.0 places humans back at the center of production processes, leveraging technology to enhance human creativity and decision-making. This transition aims to create more sustainable, resilient, and customized production systems.
For manufacturers adhering to current Good Manufacturing Practices (cGMP), this revolution is not just a technological upgrade—it’s a transformative opportunity to enhance efficiency, safety, and compliance.
What is Industry 5.0?
Industry 5.0 is the next evolutionary step in manufacturing, focusing on the seamless integration of human intelligence and advanced technology. While Industry 4.0 introduced the concept of “smart factories” with interconnected systems and autonomous machines, Industry 5.0 emphasizes the synergy between human workers and machines. This collaboration fosters innovation, precision, and customization, allowing businesses to produce high-quality products while maintaining a human touch.
Key characteristics of Industry 5.0 include:
- Human-Centric Design: Prioritizing the role of human creativity and decision-making.
- Collaborative Robotics (Cobots): Robots that work alongside humans to enhance productivity and reduce strain.
- Enhanced Customization: Meeting the growing demand for personalized products.
- Sustainability Focus: Implementing eco-friendly practices to reduce waste and energy consumption.
How Does Industry 5.0 Differ from Industry 4.0?
While both Industry 4.0 and 5.0 leverage advanced technologies, their objectives and approaches differ significantly: For example, while Industry 4.0 may utilize fully automated production lines to maximize efficiency, Industry 5.0 introduces cobots to work alongside humans, enabling personalized products such as customized pharmaceuticals or niche cosmetic formulations. This shift not only enhances flexibility but also integrates sustainability into the core of manufacturing processes.
Key Pillars of Industry 5.0 in cGMP
- Human-Centric Automation Unlike Industry 4.0, which focused on full automation, Industry 5.0 emphasizes human-machine collaboration. By integrating cobots (collaborative robots) into manufacturing processes, manufacturers can improve accuracy and reduce manual labor without sidelining human oversight.
- Personalized Manufacturing Industry 5.0 promotes greater customization, allowing manufacturers to adapt their processes to meet individualized product specifications. This is particularly valuable in industries like pharmaceuticals, where personalized medicine is gaining traction.
- Enhanced Data Utilization Leveraging AI-driven analytics and IoT devices enables real-time monitoring and predictive maintenance. For cGMP compliance, this means proactive identification of potential issues, reducing the risk of deviations and product recalls.
- Sustainability and Efficiency Industry 5.0 aligns with the global push for sustainability by optimizing resource use and reducing waste. Smart energy management systems can ensure that cGMP facilities remain energy-efficient while maintaining high production standards.
Impacts on cGMP Compliance
- Improved Documentation and Traceability Advanced software systems streamline the documentation process, ensuring that every step of production is tracked. This strengthens traceability, a core component of cGMP compliance.
- Quality by Design (QbD) Integration With AI-enabled systems, manufacturers can implement QbD more effectively. These technologies help identify critical control points, ensuring consistent product quality and reducing variability.
- Enhanced Training and Workforce Development Industry 5.0 tools can provide immersive training experiences through virtual reality (VR) and augmented reality (AR). This ensures that employees are well-equipped to operate advanced machinery and adhere to cGMP guidelines.
Challenges in Adopting Industry 5.0 for cGMP
- High Initial Investment Implementing Industry 5.0 technologies requires significant capital investment. For small and medium-sized manufacturers, this may pose a financial challenge.
- Regulatory Adaptation As technologies evolve, regulatory frameworks must adapt to ensure that new methods comply with cGMP standards. Manufacturers may face hurdles in obtaining regulatory approval for novel processes.
- Cybersecurity Risks Increased connectivity in Industry 5.0 also heightens the risk of cyberattacks. Ensuring robust data security measures is essential to protect sensitive manufacturing and compliance data.
Practical Steps for Manufacturers
- Assess Readiness and Develop a Roadmap Conduct a comprehensive audit of current processes to identify areas where Industry 5.0 technologies can bring the most value.
- Invest in Scalable Technology Start with scalable solutions, such as modular IoT devices or cloud-based analytics platforms, to minimize upfront costs while allowing for future expansion.
- Prioritize Workforce Training Equip employees with the skills needed to collaborate effectively with advanced technologies. Implement continuous learning programs to keep the workforce agile.
- Collaborate with Experts Partner with consultants and technology providers who specialize in integrating Industry 5.0 into cGMP environments. Their expertise can streamline the transition and ensure regulatory compliance.
Conclusion: Embracing the Future of Manufacturing
Industry 5.0 offers a groundbreaking opportunity for cGMP manufacturers to elevate their operations. By blending human creativity and advanced technology, businesses can achieve unprecedented levels of quality, efficiency, and sustainability. Manufacturers who proactively embrace these changes will not only stay ahead of regulatory requirements but also gain a significant competitive edge in an increasingly dynamic market.
Call to Action
Ready to explore how Industry 5.0 can revolutionize your manufacturing process? Contact us at cGMP Consulting today for a personalized roadmap to integrate cutting-edge technologies while maintaining compliance and excellence.





