cGMP Consulting streamlines Modernization of Cosmetics Regulation Act (MoCRA) compliance with tailored policies for a smooth transition.
cGMP Consulting ensures global GMP compliance through audits, PAI readiness, supplier qualification, due diligence, and gap assessments, ensuring quality and regulatory adherence.
Glove testing involves the thorough examination of isolator gloves to detect leaks, holes, and assess overall glove integrity.
The emergence of innovative digital technologies and advancements in artificial intelligence and automation is ushering in a fresh era of manufacturing innovation.
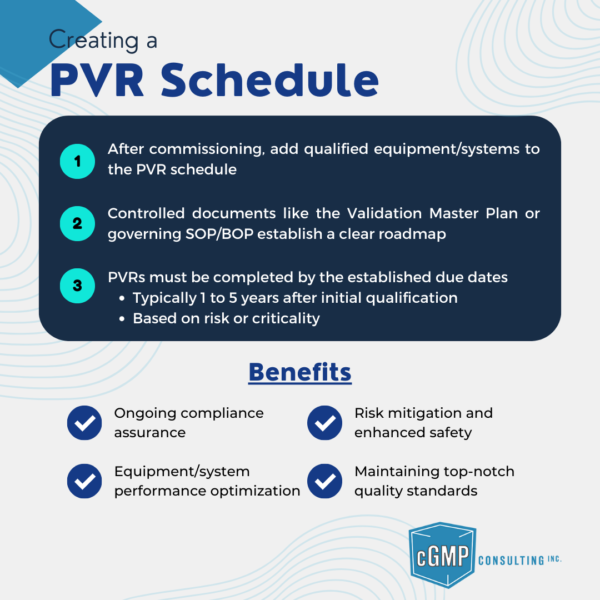
Create PVR Schedule for qualified equipment/systems in controlled documents like Validation Master Plans or SOP/BOP.
Periodic Validation Review (PVR) ensures continual control and compliance of validated systems through scheduled assessments.
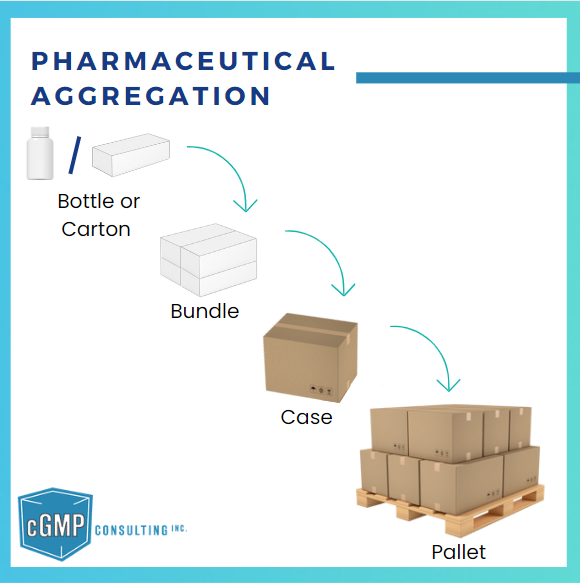
Pharmaceutical aggregation establishes a hierarchical relationship between serialized packaging levels (carton, case, or pallet).
Temperature mapping is a Good Manufacturing Practice (GMP) utilized by a variety of industries to capture how temperature is distributed within a space.
Cleanrooms are highly controlled spaces where pharmaceuticals are produced. Cleaning is the key element of contamination control.
Automated cleaning in pharmaceuticals optimizes labor efficiency, ensures consistent results, and enhances safety through PLC monitoring.