Avoiding Common FDA 483 Observations: An Industry-Specific Compliance Guide
Top FDA 483 Observations by Industry (2023)
In highly regulated industries such as pharmaceuticals, medical devices, and foods, maintaining regulatory compliance is essential. One of the key tools the U.S. Food and Drug Administration (FDA) uses to enforce compliance is the FDA 483, issued after inspections to document potential violations. These FDA 483 observations can significantly disrupt operations if not addressed.
This guide highlights the most common FDA 483 observations across top industries, the risks involved, and best practices for avoiding them.
What is an FDA 483 Observation?
An FDA 483 is a document, sometimes simply called a 483, issued after an FDA inspection if potential violations are observed to outline concerns that need corrective action.
Although it doesn’t serve as an official noncompliance ruling, failing to resolve FDA 483 observations can lead to more severe actions, such as Warning Letters, fines, or even facility shutdowns.
How to Handle an FDA 483 Response
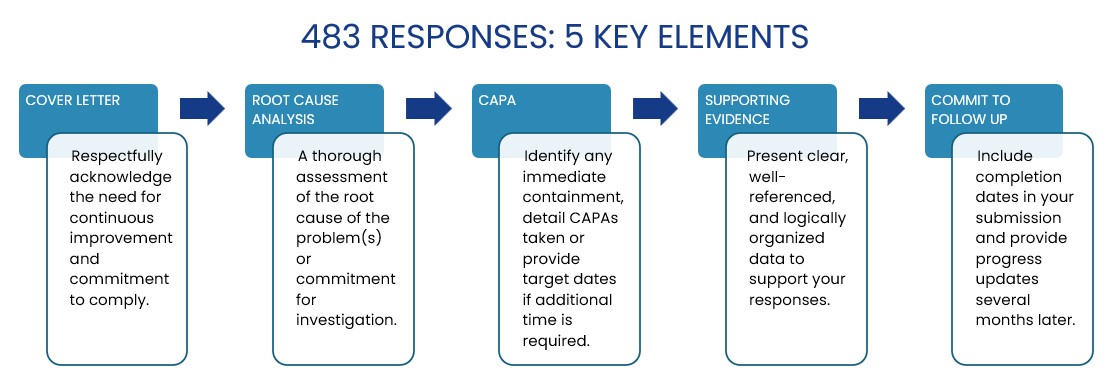
Receiving an FDA 483 can feel daunting, but with a structured response, you can effectively address each observation and demonstrate your commitment to compliance. Here are five essential elements to include in your 483 response to the FDA:
- Cover Letter: Begin with a respectful acknowledgment of the observation, expressing your commitment to continuous improvement and regulatory compliance.
- Root Cause Analysis: Conduct a thorough root cause analysis of each issue or outline your plan for an in-depth investigation if further analysis is needed.
- Corrective and Preventive Actions (CAPA): Describe any immediate containment actions taken, outline specific CAPAs, or provide target completion dates if additional time is necessary.
- Supporting Evidence: Supply clear, well-organized, and fully referenced data to back up your responses, ensuring transparency and credibility.
- Commitment to Ongoing Follow-Up: Include expected completion dates for each action, and plan to submit progress updates several months later to demonstrate sustained compliance efforts.
By addressing each of these elements thoroughly, you can show the FDA that your organization is proactive, compliant, and dedicated to quality improvement.
Number of 483s Issued by Industry
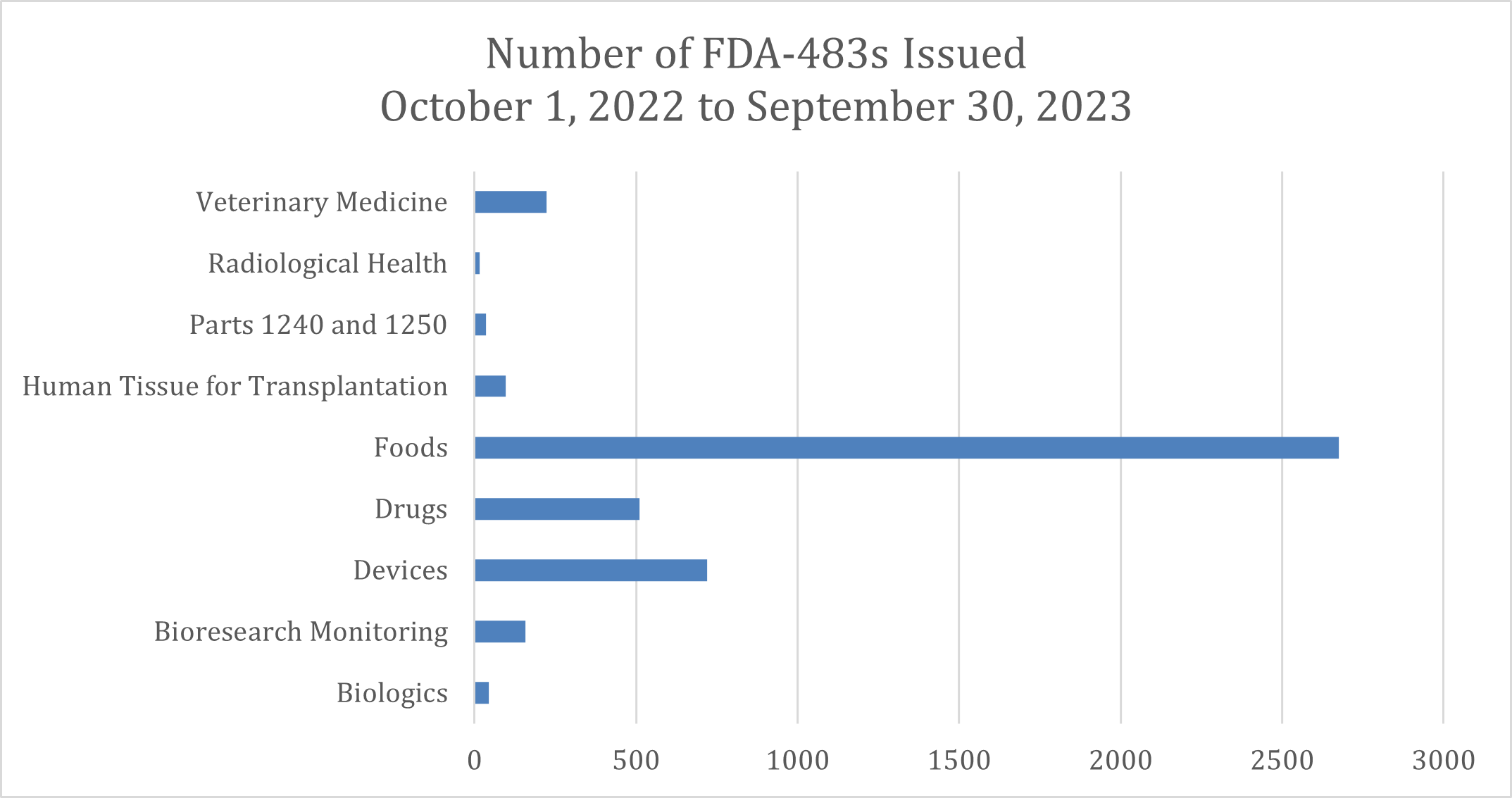
From October 1, 2022, to September 30, 2023, the FDA issued 4,481 FDA-483s across various industries. Here’s a breakdown of the top industries:
The top five industries with the most observations are as follows:
- Foods: 2,676
- Medical Devices: 720
- Pharmaceuticals: 510
- Veterinary Medicine: 224
- Bioresearch Monitoring: 158
Next, let’s explore the most common observations in each of these top industries and practical strategies to prevent them.
Top FDA 483 Observations by Industry
1. Foods
- Top Observation: Failure to develop a Foreign Supplier Verification Program (FSVP)
- Regulation: 21 CFR 1.502(a)
- Issue: Many companies lack an adequate FSVP as required under the Food Safety Modernization Act (FSMA), increasing the risk of importing unsafe products.
- Solution:
- Conduct risk assessments for all imported products to identify hazards.
- Create a written FSVP outlining steps to ensure supplier compliance.
- Regularly audit and verify supplier performance.
- Ensure staff receive FSVP training and maintain up-to-date records of compliance efforts.
2. Medical Devices
- Top Observation: Lack of or insufficient CAPA Procedures
- Regulation: 21 CFR 820.100(a)
- Issue: Medical device manufacturers are required to establish and maintain Corrective and Preventive Action (CAPA) procedures to manage nonconformities.
- Solution:
- Develop robust CAPA procedures including root cause analysis and corrective actions.
- Document all CAPA activities and ensure regular staff training to maintain compliance.
3. Pharmaceuticals
- Top Observation: Procedures not documented or followed fully
- Regulation: 21 CFR 211.22(d)
- Issue: Pharmaceutical companies must have written quality control procedures that are consistently followed. Failing to document or adhere to procedures can lead to quality deviations.
- Solution:
- Create or revise Standard Operating Procedures to make them clear, detailed, and compliant with regulations, including specific instructions for each process.
- Set up a document control system to ensure all procedures are reviewed, approved, and easily accessible, with version control and regular updates.
- Regularly perform internal audits to check compliance with procedures and identify any issues early.
- Create a Corrective and Preventive Action (CAPA) plan to address instances of non-compliance, identify root causes, and implement measures to prevent recurrence.
- Provide regular training on procedures and conduct audits to ensure adherence, highlighting the importance of compliance for product quality.
- Include compliance discussions in management reviews to ensure accountability and support from leadership.
- Establish metrics to assess the effectiveness of SOPs and training and review them regularly for continuous improvement.
4. Veterinary Medicine
- Top Observation: Unsanitary Conditions
- Regulation: FDCA 402(a)(4)
- Issue: Under FDCA 402(a)(4), products are considered adulterated if held under unsanitary conditions, posing contamination risks.
- Solution:
- Implement strict sanitation protocols including regular cleaning.
- Train staff on hygiene and conduct regular sanitation audits.
- Document all sanitation activities and corrective actions.
5. Bioresearch Monitoring
- Top Observation: Failure to follow Protocol Compliance
- Regulation: 21 CFR 312.60
- Issue: Investigators must strictly follow the investigational plan outlined in FDA Form 1572. Deviations can compromise safety and trial validity.
- Solution:
- Ensure strict adherence to protocols and provide staff training.
- Regularly review compliance and document all protocol deviations.
- Seek Institutional Review Board (IRB) approval for protocol changes and update FDA Form 1572 as necessary.
Best Practices for Avoiding FDA 483 Observations
To avoid receiving an FDA 483, organizations should implement best practices that promote a culture of quality and compliance. Key practices include:
- Proactive Compliance Programs: Continuously monitor quality control, manufacturing processes, and regulatory adherence.
- Routine Internal Audits: Regularly audit areas like record-keeping and sanitation to identify potential compliance issues.
- Proper Training: Train all employees on FDA regulations, cGMPs, and industry-specific guidelines, with refresher courses.
- Thorough Documentation: Keep meticulous records of all Standard Operating Procedures (SOPs), validation activities, and audits.
- Engage Experienced Partners: Work with compliance experts who understand FDA regulations to review your programs and identify potential gaps.
By integrating these best practices, organizations can significantly reduce the likelihood of receiving an FDA 483 and enhance their overall quality and compliance efforts.
How cGMP Consulting Can Help
At cGMP Consulting, we specialize in helping companies stay compliant and avoid 483 observations through:
- Audits: Identifying and addressing noncompliance risks.
- Qualification and Validation: Assisting in developing and executing validation protocols.
- Training Programs: Customized training on FDA regulations, best practices and audit readiness.
- Regulatory Guidance: Expertise in FDA requirements for pharmaceuticals, biotechnology, and other regulated industries.
With over 20 years of experience, we help businesses maintain compliance and minimize their risk.
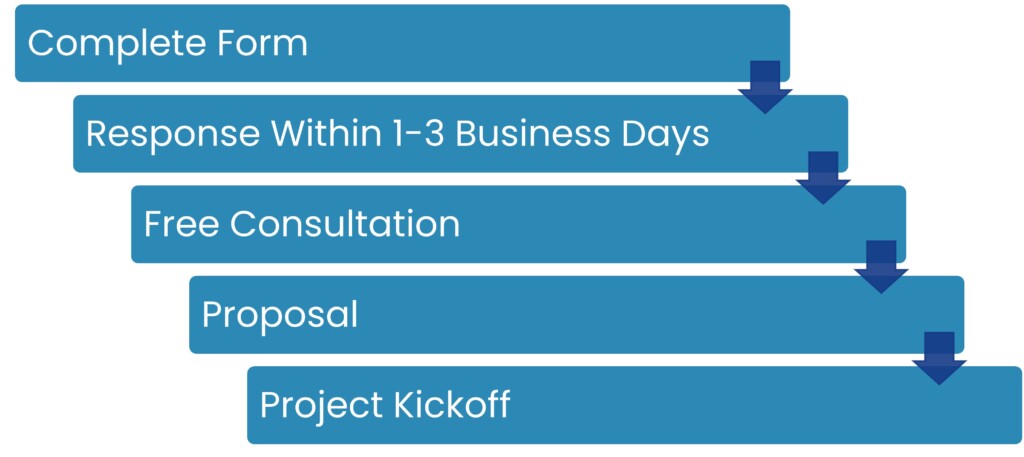
It’s easy to collaborate with cGMP Consulting. Here are five steps to get started:
- Complete Form: Fill out our Contact Us form to initiate the process.
- Response Within 1-3 Business Days: Our team will reach out to schedule your free consultation.
- Free Consultation: We discuss your needs, timeline, and budget to ensure alignment.
- Proposal: We develop a tailored plan, collaborating with you to ensure it aligns with your needs.
- Project Kickoff: Our team begins work, focused on delivering quality results.
For more information on how cGMP Consulting can proactively help you, contact us today.