Discover how Industry 5.0 revolutionizes cGMP by enhancing human-machine collaboration, improving efficiency, and ensuring compliance through advanced technology and innovation.
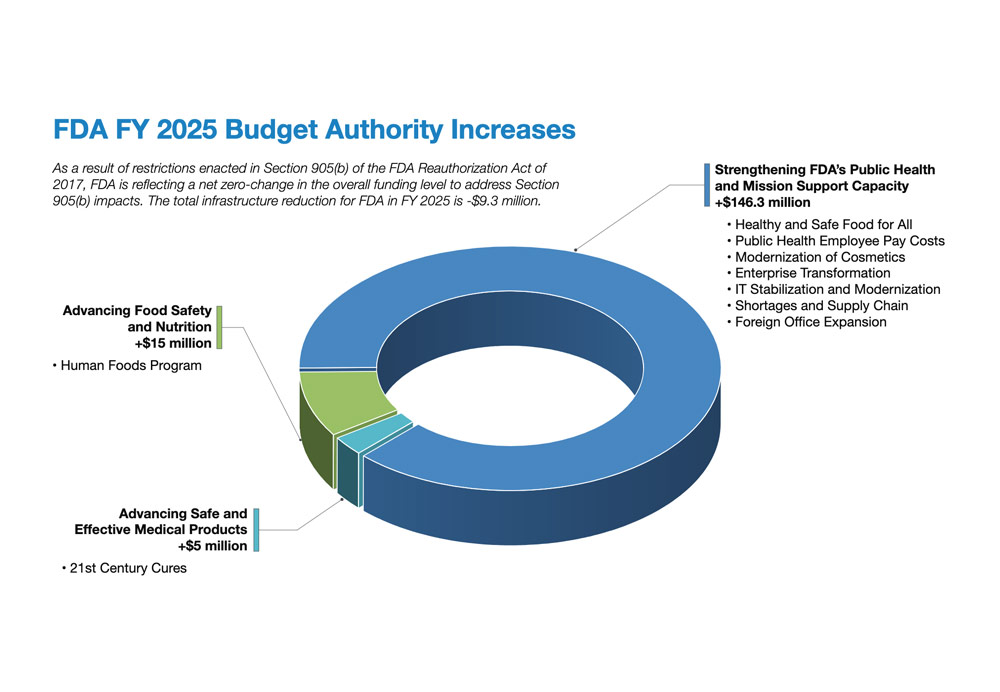
Discover the highlights of the FY 2025 FDA budget, featuring a $7.2 billion total with a $495 million increase over 2023. Learn about the FDA's funding priorities and public health initiatives.
Nexus Pharmaceuticals receives Honorable Mention at the prestigious 2023 Facility of the Year Awards (FOYA) by ISPE.
Both of our clients are finalists – Fresenius Kabi Melrose Park and Nexus Pharmaceutical at Pleasant Prairie