Discover how cGMP Consulting’s validation engineers support GMP compliance through documentation, coordination, execution, and audit-ready processes.
Explore what it takes to move from preclinical to clinical, with a focus on cGMP readiness and key challenges to be aware of for a successful transition.
Key questions to evaluate GMP certification readiness, spot gaps, and build a stronger quality system for FDA-regulated industries.
Learn how a risk-based approach streamlines facilities, utilities, and equipment qualification for smarter, compliant pharmaceutical manufacturing.
Ensure compliance with controlled room temperature qualification and temperature mapping for warehouses, freezers, and incubators.
Ensure compliance through proper facility, utility, and equipment qualification. Learn about IQ, OQ, PQ, and best practices for pharmaceutical manufacturing.
Learn about the process for ensuring controlled storage conditions, temperature mapping. Discover key equipment considerations. cGMP Consulting can help!
Learn why temperature mapping is vital for FDA-regulated distributors—ensuring compliance, product integrity, and risk protection.
Discover how to create a cross-contamination risk assessment as a part of a Contamination Control Strategy to ensure compliance and safeguard product quality.
Learn how to assess and reduce cross-contamination risks in GMP to ensure product safety and quality compliance.
Learn why equipment requalification is essential for maintaining FDA compliance and operational efficiency.
Learn the key steps and best practices to qualify sterile fill line equipment and ensure compliance.
Discover proven data integrity practices for CQV to maintain compliance in equipment qualification processes.
Optimize your equipment for GMP compliance. Our guide covers qualification steps to ensure performance, reliability, and regulatory standards
Learn about Commissioning, Qualification, and Validation (CQV) in cGMP environments to ensure compliance and product quality in the pharmaceutical industry.
Explore the benefits of vaporized hydrogen peroxide chambers for sterilization in GMP environments. Essential insights for compliant manufacturing.
Learn how cross contamination risk assessments can protect your products and ensure GMP compliance. Practical tips for every step.
Explore effective risk management strategies in regulated industries. Learn how cGMP Consulting can enhance your risk assessment processes for safety and compliance.
Ensure your gloves meet compliance standards. Explore essential glove testing procedures for GMP certification and safety in manufacturing.
Discover how to build intelligent factories with digital technologies, agile processes, and modular systems. Improve efficiency, sustainability, and adaptability in modern manufacturing.
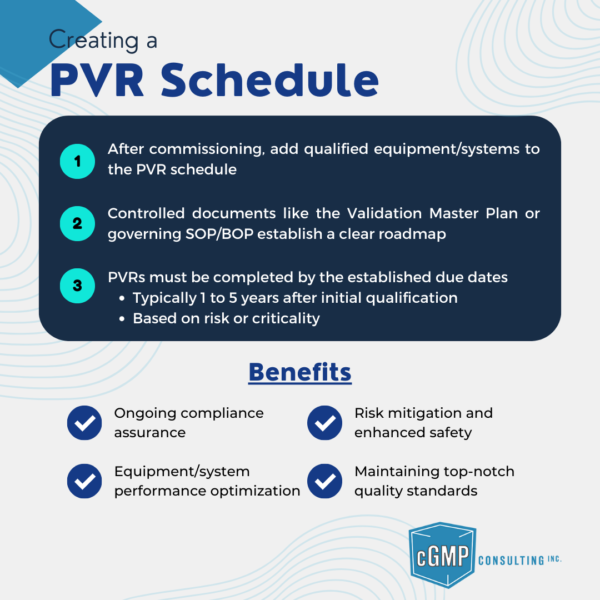
Learn how to create an effective Periodic Validation Review (PVR) Schedule for qualified equipment and systems
Discover what a Periodic Validation Review (PVR) is, how it's conducted, and how often it should be performed to ensure ongoing compliance and system control.
Temperature mapping is a Good Manufacturing Practice (GMP) utilized by a variety of industries to capture how temperature is distributed within a space.
Cleanrooms are highly controlled spaces where pharmaceuticals are produced. Cleaning is the key element of contamination control.
Automated cleaning in pharmaceuticals optimizes labor efficiency, ensures consistent results, and enhances safety through PLC monitoring.