Learn how startups and growing manufacturers can build scalable GMP systems with expert support focused on clarity, confidence, and long-term compliance.
A clear, practical look at how new manufacturers can build and use a Quality Management System that supports GMP compliance.
How new manufacturers can build a strong GMP foundation and compliance culture from day one.
Preparing to launch a product or scale production? Learn the basics of GMP and how to build quality systems early—before your first batch leaves the facility.
Learn how centerlining reduces downtime, improves product quality, and builds consistency in manufacturing for lasting efficiency and performance.
Discover how cGMP Consulting’s validation engineers support GMP compliance through documentation, coordination, execution, and audit-ready processes.
Struggling with test methods and specs? See how cGMP Consulting guides dietary supplement makers to confident compliance with 21 CFR Part 111.
Explore how project management drives successful new product introduction in the pharmaceutical industry.
Explore what it takes to move from preclinical to clinical, with a focus on cGMP readiness and key challenges to be aware of for a successful transition.
Key questions to evaluate GMP certification readiness, spot gaps, and build a stronger quality system for FDA-regulated industries.
Key questions to evaluate GMP certification readiness, spot gaps, and build a stronger quality system for FDA-regulated industries.
Learn how six key trends are reshaping dietary supplement manufacturing and 21 CFR Part 111 compliance across the industry.
Learn how a risk-based approach streamlines facilities, utilities, and equipment qualification for smarter, compliant pharmaceutical manufacturing.
Ensure compliance with controlled room temperature qualification and temperature mapping for warehouses, freezers, and incubators.
Ensure compliance through proper facility, utility, and equipment qualification. Learn about IQ, OQ, PQ, and best practices for pharmaceutical manufacturing.
Learn the top FDA observations in dietary supplement manufacturing, how to avoid these compliance issues, and how cGMP Consulting can help you meet 21 CFR Part 111.
Understand 21 CFR Part 111, the cGMP dietary supplement regulations for safe and compliant supplement manufacturing.
Learn about the process for ensuring controlled storage conditions, temperature mapping. Discover key equipment considerations. cGMP Consulting can help!
Learn why temperature mapping is vital for FDA-regulated distributors—ensuring compliance, product integrity, and risk protection.
Discover how cGMP compliance helps distributors access regulated industry opportunities by ensuring quality and building trust. Let cGMP Consulting help!
Discover how to create a cross-contamination risk assessment as a part of a Contamination Control Strategy to ensure compliance and safeguard product quality.
Learn key steps to conduct effective internal audits, ensure FDA compliance, and boost operational efficiency in 2025. Stay ahead with expert tips.
Discover how Industry 5.0 revolutionizes cGMP by enhancing human-machine collaboration, improving efficiency, and ensuring compliance through advanced technology and innovation.
Explore GMP vs. cGMP, their key differences, principles, and benefits, and understand why staying 'current' is essential for regulatory compliance and manufacturing success.
Learn the key differences between GMP and cGMP, why compliance matters, and how to implement cGMP for regulatory success. Stay competitive and compliant with this essential guide.
Get essential insights on FDA and GMP compliance to help your business meet regulatory standards effectively.
This guide covers the fundamentals of Good Manufacturing Practices (GMP), the 10 key elements for compliance, the GMP process, best practices, and FDA inspection insights.
Learn how a food and OTC manufacturer successfully resolved an FDA warning letter and improved their compliance practices.
Learn how to assess and reduce cross-contamination risks in GMP to ensure product safety and quality compliance.
Discover practical strategies to avoid FDA 483 observations and ensure seamless compliance in your industry.
Learn why equipment requalification is essential for maintaining FDA compliance and operational efficiency.
Learn the key steps and best practices to qualify sterile fill line equipment and ensure compliance.
Prevent FDA 483 issues with expert strategies in compliance, data integrity, and quality systems from cGMP Consulting.
Discover proven data integrity practices for CQV to maintain compliance in equipment qualification processes.
Understand the financial and operational risks of receiving an FDA 483 and how to prevent compliance issues.
Optimize your equipment for GMP compliance. Our guide covers qualification steps to ensure performance, reliability, and regulatory standards
Discover immediate actions to take after receiving an FDA 483 and prevent further compliance issues.
Prepare your organization for success with cGMP Consulting's Audit Readiness Training. Our 5-step process equips your team to excel in regulatory inspections.
Learn about Commissioning, Qualification, and Validation (CQV) in cGMP environments to ensure compliance and product quality in the pharmaceutical industry.
Prepare for MoCRA GMP compliance with cGMP Consulting. Get expert help with assessments, SOPs, and training for early compliance and reduced risks.
Discover how to seamlessly implement electronic GMP systems with expert consulting from cGMP Consulting and amni.ai. Enhance compliance and operational efficiency in FDA-regulated industries.
Explore the essential elements of cosmetic GMPs and learn how cGMP Consulting can help ensure the safety, efficacy, and quality of your cosmetic products while navigating regulatory compliance.
Your essential guide to MoCRA compliance. Discover the latest regulations and steps to ensure your cosmetics meet FDA standards. Start preparing today!
Master the GMP certification process with cGMP Consulting’s ultimate guide. Learn about our comprehensive evaluation, certification recommendations, and ongoing compliance support.
Facing challenges in GMP certification? Learn about the top obstacles and expert tips to achieve compliance in regulated industries.
Elevate your quality standards with cGMP Consulting's GMP Certification Audits. ISO/IEC 17020:2012 accredited, we offer tailored audits for compliance and quality assurance.
Discover the benefits and challenges of assisted line clearance in manufacturing. Learn how automation can enhance efficiency, quality, and compliance.
Explore the benefits of vaporized hydrogen peroxide chambers for sterilization in GMP environments. Essential insights for compliant manufacturing.
Master GMP compliance with cGMP Consulting's comprehensive auditing services. Explore 8 tailored solutions to enhance product quality, ensure regulatory adherence, and build market trust.
Learn best practices for managing deviations in technical writing to ensure compliance and maintain quality standards. Essential for regulated industries.
Discover effective strategies for managing deviations in regulated industries. Learn how cGMP Consulting can help improve compliance and operational efficiency.
Learn how cross contamination risk assessments can protect your products and ensure GMP compliance. Practical tips for every step.
Explore effective risk management strategies in regulated industries. Learn how cGMP Consulting can enhance your risk assessment processes for safety and compliance.
Ensure your gloves meet compliance standards. Explore essential glove testing procedures for GMP certification and safety in manufacturing.
Discover how to build intelligent factories with digital technologies, agile processes, and modular systems. Improve efficiency, sustainability, and adaptability in modern manufacturing.
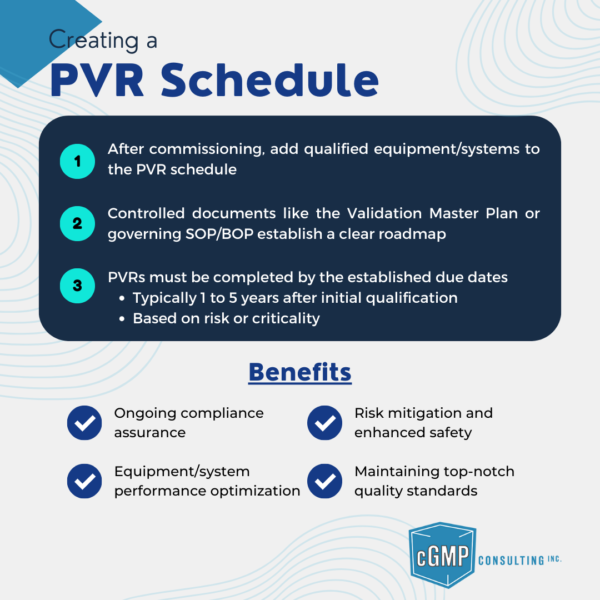
Learn how to create an effective Periodic Validation Review (PVR) Schedule for qualified equipment and systems
Discover what a Periodic Validation Review (PVR) is, how it's conducted, and how often it should be performed to ensure ongoing compliance and system control.
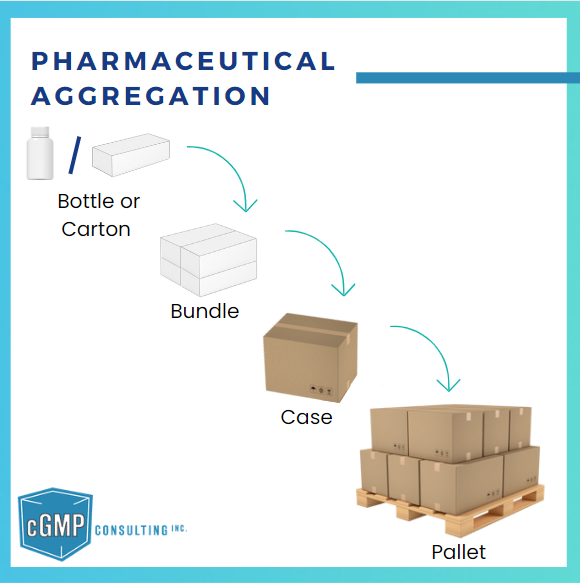
Learn how pharmaceutical aggregation establishes traceability in the supply chain by linking serialized packaging.
Temperature mapping is a Good Manufacturing Practice (GMP) utilized by a variety of industries to capture how temperature is distributed within a space.
Cleanrooms are highly controlled spaces where pharmaceuticals are produced. Cleaning is the key element of contamination control.
Automated cleaning in pharmaceuticals optimizes labor efficiency, ensures consistent results, and enhances safety through PLC monitoring.