
Success Story: Resolving an FDA Warning Letter for a Food & OTC Manufacturer
Background
A prominent food and over-the-counter (OTC) drug manufacturer approached us with an urgent request: they had received a Warning Letter from the FDA. The letter cited three critical violations, putting the company at risk of severe regulatory consequences if not promptly and adequately addressed.
The Violations Cited by the FDA:
- Component Identity Verification Test Not Conducted
The manufacturer had not conducted adequate identity verification tests for components, and the reliability of the supplier’s test analysis had not been validated. - Production and Process Control Procedures Not Written
There were no documented procedures in place to ensure consistent production and process control, resulting in potential inconsistencies. - Quality Control Unit Responsibility
The responsibilities of the Quality Control unit were not adequately defined, leading to gaps in oversight and regulatory compliance.
Given the urgency of the situation and the 15-day response window mandated by the FDA, immediate action was required. We assigned a cGMP (current Good Manufacturing Practice) Expert and a Quality Expert to spearhead the remediation efforts. Both brought extensive experience in addressing compliance issues and communicating effectively with the FDA.
Compliance Response Planning
To meet the tight timeline, we conducted daily meetings with the client’s team, focusing on clear, actionable steps. We promptly identified the critical areas of concern and outlined a structured, comprehensive plan to address each violation detailed in the Warning Letter.
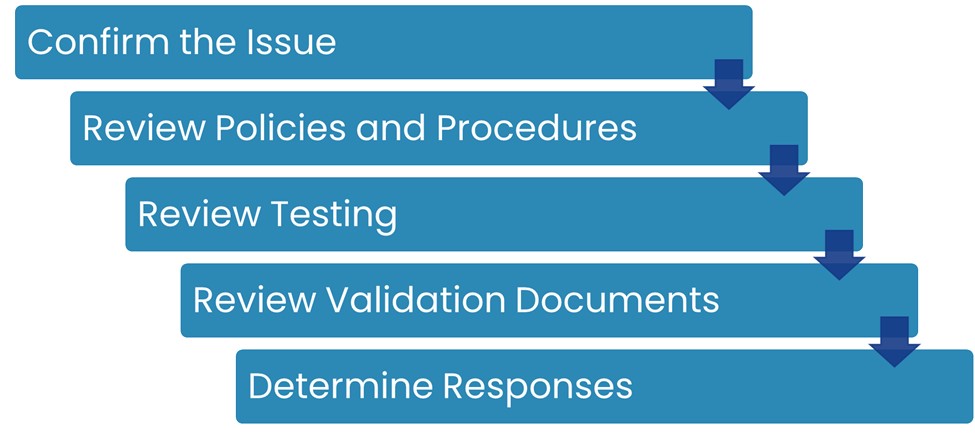
To develop an appropriate response letter, we followed a structured process:
- Confirm the Issue
- Validation of Findings: Confirmed the accuracy of the violations described in the FDA Warning Letter.
- Scope Assessment: Identified if additional areas were impacted by the cited issues and ensured the description was complete and precise.
- Review Applicable Policies and Procedures
- Policy and Procedure Evaluation: Assessed existing policies and procedures to determine if they were present and aligned with operational practices.
- Alignment Check: Verified that current processes matched documented policies and procedures.
- Policy Updates: Determined necessary updates to policies and procedures to align with best practices and compliance requirements.
- Review Testing
- Assessment of Testing Protocols: Evaluated whether relevant testing was being conducted.
- Testing Improvements: Identified enhancements to existing testing methods and ensured appropriate updates to meet regulatory expectations.
- Review Validation Documents
- Validation Verification: Checked if the required validation processes were completed and reviewed their adequacy.
- Validation Adequacy: Ensured that validation documentation was thorough and compliant with regulatory standards.
- Determine the Appropriate Responses
- Calibration: Evaluated if the proposed response actions were proportionate — ensuring they were neither excessive nor insufficient.
- Implementation: Assessed the timing of the planned responses, ensuring they were timely but not overly conservative.
- Resources: Determined if the implementation required capital investments or additional resources to execute effectively.
Through this collaborative approach and our in-depth regulatory expertise, the client was able to submit a comprehensive response to the FDA within the required 15-day timeframe. The response was accepted by the FDA without any further escalation, allowing the manufacturer to maintain operations uninterrupted.
Remediations
Following submission of the response, we acted on the corrective actions that were outlined in the response letter. Here are the details of the remediations:
Key Steps Taken:
- Materials Management Program
We established a robust Materials Management Program to address the first violation related to component identity verification. This program included:- Risk Assessment: Evaluated potential risks in the supply chain and raw material handling.
- Materials Management/IQS Development: Implemented an Integrated Quality System (IQS) for better tracking and management of raw materials.
- Ingredient Specification Development: Defined detailed specifications for chemical and microbial components to ensure consistency and safety.
- Manufacturing Operations
To address the lack of documented production and process controls, we developed critical manufacturing protocols:- Created the Master Manufacturing Record and Batch Production Records, including In-Process (IP) testing guidelines.
- Established a comprehensive Process Validation Program and introduced an Annual Product Review Program for ongoing assessment and compliance.
- Developed detailed Specification Guidelines for all product components
- Ensured thorough Cleaning Validation to prevent cross-contamination.
- Batch Record Review and Lot Release Controls
We implemented strict controls to strengthen the Quality Control Unit’s responsibilities and streamline the product release process:- Developed a robust Batch Record Review process to ensure compliance before product release.
- Introduced an Out of Specification (OOS) Procedure to handle deviations promptly and effectively.
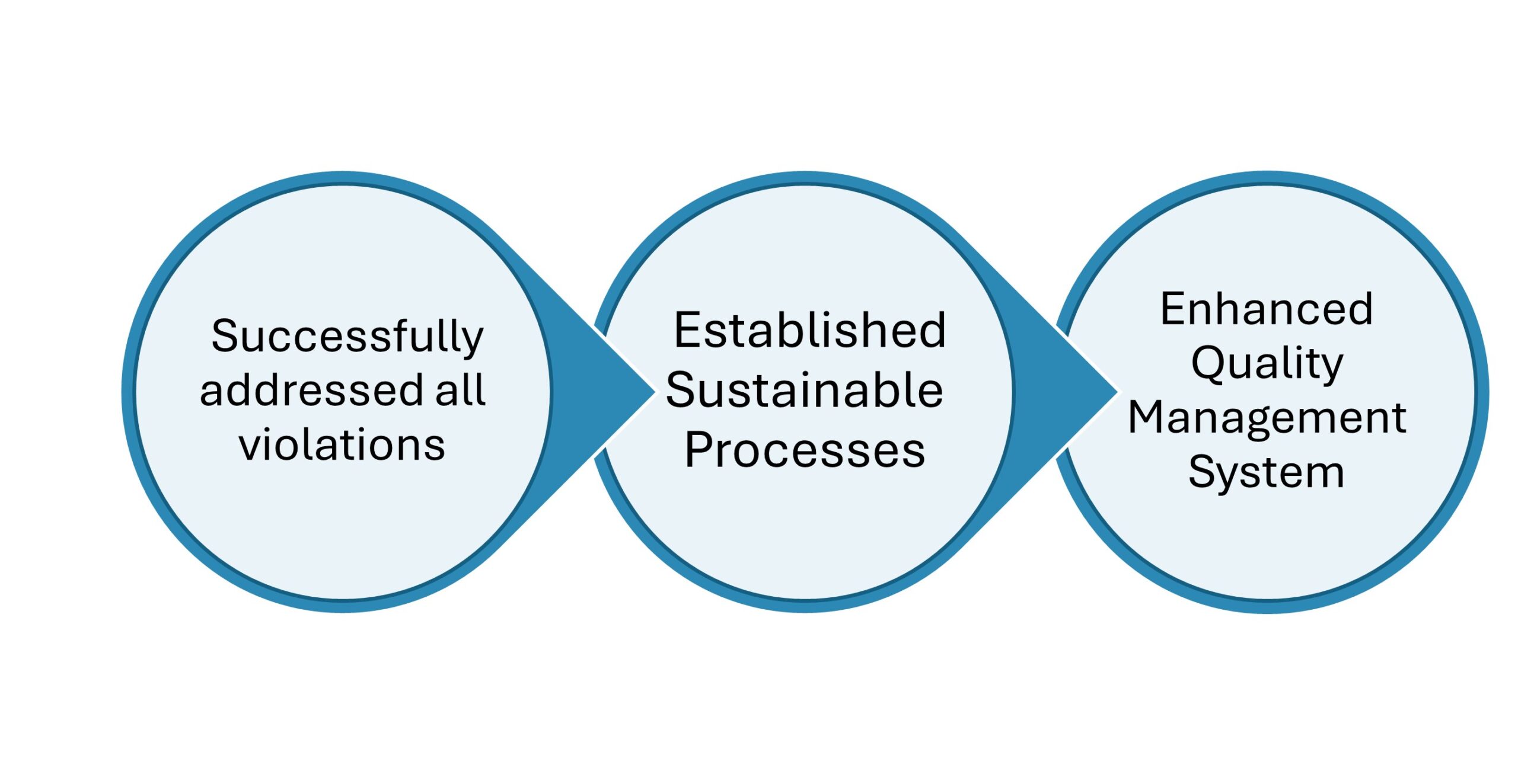
Key Outcomes:
- Successfully addressed all violations cited in the Warning Letter that satisfied the FDA.
- Established sustainable compliance processes, reducing the risk of future regulatory issues.
- Enhanced the client’s quality management systems, leading to improved product safety and consistency.
Conclusion
This case outlines the importance of a timely, strategic response when faced with regulatory challenges. Our team’s expertise, combined with close collaboration with the client, ensured a positive outcome. By implementing robust quality systems and clear procedural documentation, we not only resolved the immediate compliance issues but also set the client on a path for long-term regulatory success.
Contact us today for a consultation and let us guide you through FDA compliance to avoid future penalties and ensure your products meet the highest quality standards.






