
GMP vs cGMP: Understanding the Differences and Why They Matter in Modern Manufacturing
In FDA-regulated industries such as pharmaceuticals, biotechnology, cosmetics, and food, manufacturing practices must comply with stringent FDA compliance requirements to ensure product safety, efficacy, and quality. At the heart of these regulatory guidelines are two critical frameworks: Good Manufacturing Practices (GMP) and Current Good Manufacturing Practices (cGMP). While both are designed to uphold quality assurance and consumer safety, their approaches differ significantly. GMP establishes a foundational framework for consistent manufacturing processes, whereas cGMP emphasizes continuous improvement, modern technologies, and alignment with the latest regulatory guidelines.
This article offers a detailed comparison of GMP and cGMP, exploring their differences, principles, and why staying “current” is essential to maintaining compliance in today’s fast-evolving manufacturing landscape.
What is GMP?
Good Manufacturing Practices (GMP) are a set of internationally recognized guidelines that ensure the consistent production and quality control of products across industries such as pharmaceuticals, biotechnology, cosmetics, and food. These practices govern essential aspects of manufacturing, including personnel, facilities, equipment, processes, and documentation, creating a structured approach to compliance.
The primary goals of GMP are to:
- Prevent contamination at all stages of production.
- Minimize errors in manufacturing, packaging, and labeling.
- Ensure product consistency to meet safety and efficacy standards.
At the core of GMP compliance are the 5 Ps of GMP:
- People: Proper training and hygiene to reduce human error and contamination risks.
- Premises: Well-maintained facilities designed to support safe production.
- Processes: Standardized workflows validated for consistency and reliability.
- Products: Quality ingredients and end products that meet regulatory standards.
- Procedures: Clear documentation and protocols to guide every step of manufacturing.
In the pharmaceutical industry, adhering to GMP is critical for FDA compliance and consumer trust. Key examples include:
- Hygiene protocols to prevent microbial contamination during drug production.
- Validated cleaning procedures to avoid cross-contamination between batches of different drugs.
- Batch records for complete traceability, ensuring accountability at every stage of production.
By following GMP guidelines, manufacturers safeguard both consumer health and their own reputations, meeting stringent regulatory requirements and building trust in their products.
What is cGMP?
Current Good Manufacturing Practices (cGMP) elevate the foundational principles of GMP by integrating modern technologies, advanced methodologies, and a commitment to continuous improvement. The emphasis on “current” reflects the need for businesses to regularly update their practices to align with evolving industry standards, regulatory requirements, and technological advancements.
Key features of cGMP include:
- Real-time data monitoring to identify and resolve quality issues immediately.
- Automated systems that enhance accuracy, reduce human error, and streamline processes.
- Electronic batch records (EBRs) to improve traceability, compliance, and audit readiness.
In the pharmaceutical industry, cGMP compliance is essential to meeting FDA regulatory standards and maintaining product safety. Examples of cGMP in action include:
- Cleanroom monitoring: Real-time environmental monitoring ensures sterility during the production of sterile injectable drugs.
- Automated inspection systems: Advanced technology detects defects in tablets, such as chips or incorrect labeling, before distribution.
- Advanced validation techniques: Rigorous validation of manufacturing processes ensures consistent drug formulation and efficacy.
By adopting cGMP, businesses not only achieve regulatory compliance but also enhance operational efficiency, product quality, and consumer trust. Staying “current” is not just a recommendation—it’s a necessity in today’s fast-evolving pharmaceutical landscape.
Key Differences Between GMP and cGMP
Understanding the differences between Good Manufacturing Practices (GMP) and Current Good Manufacturing Practices (cGMP) is crucial for navigating regulatory compliance and ensuring manufacturing excellence. While GMP provides a solid foundation, cGMP takes a forward-thinking approach, incorporating modern tools and proactive strategies. Below is a detailed comparison to help you better understand their distinctions:
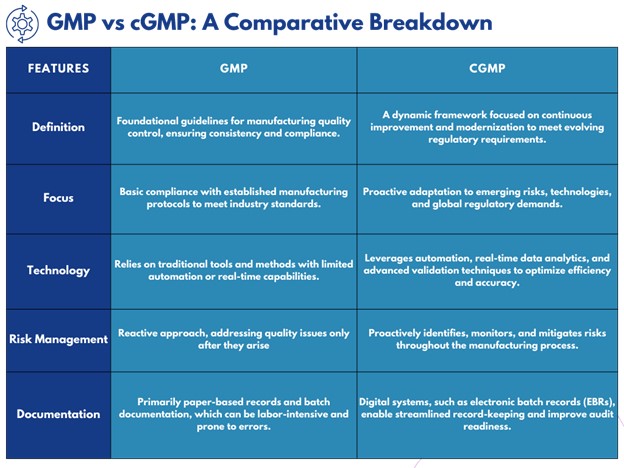
Examples of GMP and cGMP in Pharmaceutical Practices
Imagine a mid-sized pharmaceutical company producing over-the-counter pain relief tablets. Initially, the company operates under GMP guidelines, ensuring basic compliance and maintaining product quality. However, as the demand for their products grows and regulators impose stricter standards, they transition to cGMP to modernize operations and improve efficiency.
GMP in Action:
Under GMP, the company focuses on foundational quality practices:
- Batch Documentation: Each production batch is tracked using paper-based records, requiring significant manual input and review.
- Tablet Compression Validation: The compression process is manually monitored to ensure each tablet contains the correct dosage of active ingredients.
- Equipment Cleaning Protocols: Equipment is cleaned between batches to prevent cross-contamination, with cleaning processes documented and verified through routine inspections.

While GMP ensures compliance, manual processes can be labor-intensive and increase the likelihood of human error. Transitioning to cGMP can streamline these workflows and improve efficiency.
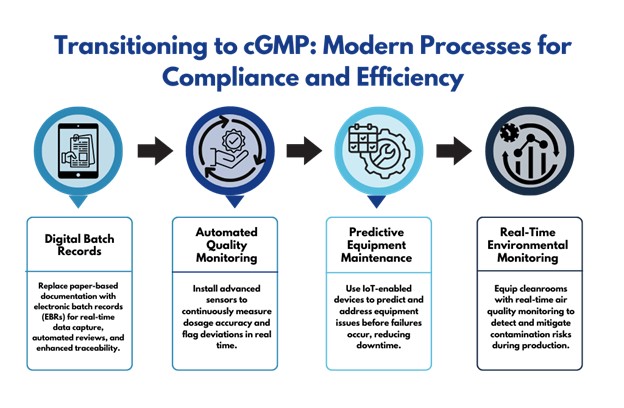
Transitioning to cGMP:
To meet growing demand and maintain competitive advantage, the company adopts cGMP, transforming its processes:
- Digital Batch Records: Paper-based documentation is replaced with electronic batch records (EBRs), enabling real-time data capture, automated review, and easier traceability.
- Automated Quality Monitoring: Advanced sensors are installed on tablet compression machines to continuously measure dosage accuracy and flag any deviations in real time.
- Predictive Equipment Maintenance: Using IoT-enabled devices, the company predicts and addresses maintenance needs before equipment failures occur, minimizing downtime and ensuring consistent quality.
- Real-Time Environmental Monitoring: Cleanrooms are equipped with air quality sensors that alert staff to potential contamination risks, ensuring sterility during production.
Outcome of cGMP Implementation:
The transition to cGMP not only improves compliance but also results in tangible benefits:
- Production times decrease by 20% due to automated processes.
- Product recalls are minimized as real-time monitoring catches issues early.
- The company expands into international markets by meeting stringent global regulatory standards.
By moving from GMP to cGMP, this pharmaceutical company exemplifies how adopting modern practices can enhance efficiency, ensure product quality, and maintain regulatory compliance in a competitive industry
Benefits of Adopting cGMP Over GMP
While Good Manufacturing Practices (GMP) establish a strong foundation for quality assurance, transitioning to Current Good Manufacturing Practices (cGMP) provides businesses with significant advantages that go beyond compliance. By integrating advanced technologies and proactive strategies, cGMP helps companies remain competitive in today’s demanding markets.
- Enhanced Operational Efficiency
Automated systems and real-time monitoring streamline production processes, reducing human error and minimizing waste. For example, a pharmaceutical manufacturer using automated tablet inspection systems can detect defects immediately, preventing costly recalls and ensuring faster production cycles. Predictive maintenance tools further reduce downtime by addressing equipment issues before they escalate. - Improved Product Consistency
cGMP ensures that every batch meets stringent quality standards. In industries like pharmaceuticals, where dosage accuracy can directly impact patient safety, this consistency is critical. Real-time analytics and advanced validation methods eliminate variability, ensuring uniformity across batches and bolstering consumer confidence. - Regulatory Readiness
Adherence to cGMP simplifies compliance with FDA regulations and global standards, making it easier to enter international markets. For instance, a company transitioning to cGMP can quickly respond to audits with electronic batch records (EBRs) and real-time quality data, demonstrating robust compliance and operational transparency. - Consumer Trust and Brand Reputation
cGMP compliance showcases a business’s commitment to quality, safety, and innovation. This not only builds long-term consumer trust but also strengthens the company’s reputation in competitive markets. For example, a biopharmaceutical company that adopts cGMP can confidently market its products as being manufactured under the highest safety and quality standards, gaining an edge in a crowded industry.
By moving beyond GMP to embrace cGMP, businesses not only meet today’s stringent regulatory requirements but also unlock opportunities for growth, efficiency, and consumer loyalty. Whether it’s reducing operational risks or entering new markets, cGMP equips companies with the tools needed to thrive in a fast-evolving regulatory landscape.
The Importance of Staying Current
In today’s fast-paced industries, the dynamic regulatory landscape demands a proactive and forward-looking approach. Adopting Current Good Manufacturing Practices (cGMP) ensures businesses remain adaptable, competitive, and compliant as new challenges and technologies emerge.
Staying “current” is critical for businesses to:
- Address Emerging Risks: Proactively identify and mitigate risks such as contamination, deviations, or supply chain disruptions before they impact product quality or consumer safety.
- Leverage Technological Advancements: Integrate cutting-edge tools like AI-driven quality control to enhance precision and efficiency or blockchain technology for transparent and secure supply chain traceability.
- Meet Evolving Consumer Expectations: Adapt to rising demands for sustainability by implementing eco-friendly manufacturing processes and using biodegradable packaging materials.
A Real-World Example: Personalized Medicine
The rise of personalized medicine—such as CAR-T cell therapies—has introduced unique compliance challenges. These therapies are manufactured for individual patients, requiring:
- Stringent environmental controls to maintain sterility and product integrity.
- Robust batch documentation to ensure traceability and adherence to regulatory standards.
cGMP enables businesses to meet these challenges by leveraging advanced monitoring systems, automated processes, and continuous quality improvements. For instance, in the production of CAR-T therapies, real-time analytics ensure cell modifications are precise, while digital records track every step of the process to meet FDA and global compliance requirements.
By staying “current” with cGMP, businesses not only navigate the complexities of modern manufacturing but also build trust with consumers and regulators, paving the way for sustainable growth and innovation.
Challenges in Transitioning from GMP to cGMP
Transitioning from Good Manufacturing Practices (GMP) to Current Good Manufacturing Practices (cGMP) is a critical step toward modernization and compliance, but it is not without its challenges. Key obstacles include:
- Cost of Modernization: Upgrading facilities, implementing new equipment, and adopting advanced software often require significant financial investment, particularly for small to mid-sized businesses.
- Employee Resistance: Introducing new workflows and technologies can lead to pushback if employees are not adequately trained or informed about the benefits of the transition.
- Global Supply Chain Management: Coordinating compliance across international suppliers adds complexity, with varying local regulations and capabilities creating potential gaps in quality management.
Overcoming These Challenges
Despite these hurdles, businesses can navigate the transition successfully by:
- Breaking the Process into Phases: Prioritize high-impact areas, such as automated quality monitoring or digital documentation, and implement changes incrementally to minimize disruption.
- Investing in Ongoing Employee Training: Foster a culture of accountability and engagement by providing comprehensive, role-specific training that highlights the benefits of cGMP.
- Partnering with Industry Experts: Leverage the expertise of seasoned cGMP consultants to streamline the implementation process, identify potential pitfalls, and ensure compliance with FDA and global standards.
Which is Right for Your Business?
While GMP establishes a strong foundation for regulatory compliance, it may not be enough to keep pace with the demands of today’s fast-evolving industries. cGMP offers the tools and strategies needed to modernize operations, enhance efficiency, and meet global regulatory requirements. For businesses seeking long-term growth, innovation, and consumer trust, transitioning to cGMP is not just an option—it’s a necessity.
Ready to Take Your Manufacturing to the Next Level?
The journey to cGMP compliance can be challenging, but you don’t have to navigate it alone. Our team of experts is here to help you implement tailored strategies that align with your business needs and regulatory requirements.
Contact us today to schedule a free consultation and take the first step toward building a compliant, efficient, and competitive future. Let’s transform your operations and set your business apart in today’s dynamic market.
“This post was developed with input from our GMP experts and crafted by our SEO and blogging specialist, Tayler Awad, to enhance accessibility and online reach.”





