A Comprehensive Guide to Good Manufacturing Practices (GMP)
Good Manufacturing Practices (GMP), also referred to as current Good Manufacturing Practices (cGMP), are the cornerstone of quality assurance in manufacturing industries such as pharmaceuticals, biotechnology, food, and cosmetics. These guidelines, enforced by regulatory bodies like the FDA, ensure that products meet the highest standards of safety, quality, and consistency.
Whether you’re a new business navigating compliance requirements or an established company looking to refine processes, this guide will help you understand the significance of GMP and how to implement it effectively.
What is GMP and Why is it Important?
Good Manufacturing Practices (GMP) refer to a system of guidelines that ensure products are consistently produced and controlled according to quality standards. These practices cover every aspect of the manufacturing process, from raw materials and facilities to equipment, training, and documentation.
GMP aims to minimize the risks associated with manufacturing. These risks include contamination, errors in labeling, and variations in product quality, all of which could harm consumers or damage a company’s reputation.
What Are the GMP Requirements? The 10 Key Elements
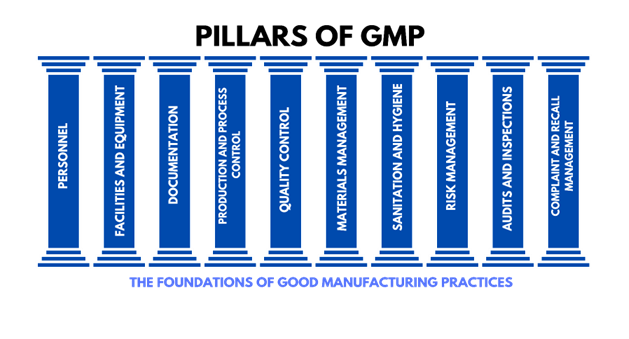
Good Manufacturing Practices (GMP) are essential principles designed to ensure that products are consistently produced and controlled to meet high-quality standards. These elements play a critical role in maintaining product safety, efficacy, and adherence to regulatory requirements. Below is a comprehensive overview of the key components of GMP compliance:
1) Personnel
Training: Employees must be adequately trained in GMP principles and their specific roles.
Hygiene: Strict personal cleanliness and health checks are necessary to prevent contamination.
Competency: Staff should possess the qualifications, skills, and experience required for their responsibilities.
2) Facilities and Equipment
Design: Facilities must minimize contamination risks and enable proper cleaning and maintenance.
Maintenance: Equipment should undergo routine calibration, cleaning, and upkeep.
Environment: Maintain controlled conditions for temperature, humidity, and cleanliness as required by the product.
3) Documentation
Standard Operating Procedures (SOPs): Clearly written procedures for all processes and practices.
Batch Records: Complete records for every batch to ensure traceability.
Change Control: Documentation of any modifications to processes, equipment, or systems.
Training Records: Proof that employees are trained and competent.
4) Production and Process Control
Validation: Processes must be validated to ensure consistency and reliability.
Monitoring: Regular monitoring and control of production processes to maintain standards.
Deviation Management: Procedures to identify, document, and address deviations from standards.
5) Quality Control
Testing: Raw materials, intermediates, and finished products must be tested to meet specifications.
Release: Products should only be released for sale after thorough quality checks.
Stability Studies: Ongoing testing to ensure product quality over time.
6) Materials Management
Supplier Qualification: Ensure that raw materials come from approved suppliers.
Storage: Proper storage conditions to avoid contamination and deterioration.
Inventory Control: Implement FIFO (First-In, First-Out) or FEFO (First-Expired, First-Out) systems for material handling.
7) Sanitation and Hygiene
Cleaning Procedures: Detailed cleaning protocols for equipment, facilities, and personnel.
Pest Control: Prevent contamination from pests through proactive measures.
Waste Management: Proper disposal systems for waste to avoid cross-contamination.
8) Risk Management
Hazard Analysis: Identify and assess risks in processes, materials, and environments.
Corrective Actions: Plans to address risks and prevent recurrence of issues.
Preventive Measures: Strategies to avoid potential quality or safety issues.
9) Audits and InspectionsInternal Audits: Regular self-inspections to identify and correct GMP gaps.
Regulatory Readiness: Be prepared for inspections by authorities like the FDA or EMA.
Continuous Improvement: Use audit findings to refine processes and systems.
10) Complaint and Recall Management
Complaint Handling: Systems to address customer complaints, investigate issues, and document actions.
Product Recall: Plans to quickly and effectively remove unsafe or non-compliant products from the market.
The key elements of GMP ensure that every step of the production process aligns with regulatory requirements, minimizes risks, and guarantees product quality and safety. Implementing these principles is essential for maintaining compliance and protecting consumer trust.
Why GMP Compliance is Critical
- Consumer Safety
GMP guidelines ensure that products are free from contaminants and safe for their intended use. This is especially vital in industries like pharmaceuticals, where even minor errors can have life-threatening consequences. - Quality and Consistency
GMP enforces standardized procedures, ensuring that every product meets the same quality benchmarks. Consistency is key, particularly for drugs and medical devices where efficacy depends on uniformity. - Regulatory Compliance
Adherence to GMP is a legal requirement in FDA-regulated industries. Non-compliance can lead to warnings, fines, product recalls, or even shutdowns. - Market Reputation and Growth
Companies that consistently follow GMP gain a competitive edge, building trust with consumers, regulators, and business partners. This can pave the way for expansion into global markets. - Reduced Liability Risks
Following GMP guidelines minimizes the chances of defective products reaching consumers, reducing legal exposure and protecting the brand.
What Are the 5 Ps of GMP?
The “5 Ps of GMP” refer to People, Premises, Processes, Products, and Procedures—the key elements that ensure compliance with Current Good Manufacturing Practices. These components are essential for maintaining the quality, safety, and consistency of products in FDA-regulated industries.
- People emphasize the importance of training and qualifications, ensuring that personnel are equipped to follow and implement GMP standards.
- Premises focus on maintaining clean, organized, and properly designed facilities to prevent contamination and ensure operational efficiency.
- Processes highlight the need for validated, standardized workflows to guarantee consistent product quality.
- Products underscore the importance of quality raw materials and finished goods that meet defined specifications.
- Procedures are the documented guidelines (SOPs) that govern every stage of manufacturing, from production to quality control.
By addressing each of these Ps, manufacturers can build a robust framework for cGMP compliance, ensuring products meet both regulatory and consumer expectations.
Breaking Down the GMP Process
A robust GMP process is essential for ensuring that every product meets the highest standards of quality, safety, and efficacy. It involves a series of interconnected stages, each playing a critical role in compliance. Here’s a detailed breakdown:
1. Raw Material Procurement
The foundation of quality begins with the raw materials:
- Supplier Compliance: Source materials only from approved suppliers who meet strict quality standards and GMP requirements.
- Material Testing: Conduct thorough testing to ensure raw materials meet predefined specifications, such as purity, potency, and absence of contaminants.
- Traceability: Maintain detailed records of raw material origins and test results to ensure accountability and enable quick recalls if necessary.
2. Manufacturing
This stage ensures the transformation of raw materials into finished products through validated processes:
- Process Validation: Develop and implement manufacturing processes that are scientifically validated to produce consistent results.
- Environmental Monitoring: Regularly monitor critical parameters, such as temperature, humidity, and cleanliness, to prevent contamination and maintain product integrity.
- Personnel Hygiene: Enforce strict hygiene protocols for personnel to minimize the risk of contamination during production.
3. Quality Control (QC)
Quality control ensures that every product meets safety and efficacy standards before reaching consumers:
- In-Process Testing: Perform tests during manufacturing to detect and address deviations early. Examples include weight variation, pH testing, and microbiological assessments.
- Finished Product Testing: Analyze the final product for attributes such as potency, purity, and stability.
- Stability Testing: Conduct long-term and accelerated stability studies to confirm shelf life and storage conditions.
4. Packaging and Labeling
Proper packaging and labeling are critical to preserving product quality and ensuring accurate information delivery:
- Contamination Prevention: Use packaging materials designed to protect the product from contamination, moisture, light, or other environmental factors.
- Accurate Labeling: Verify that all labels include correct product information, such as dosage instructions, batch numbers, expiration dates, and regulatory compliance statements.
- Tamper-Evident Features: Incorporate tamper-evident seals to enhance consumer safety and confidence.
5. Distribution
The final step involves ensuring products reach consumers safely and in compliance with regulations:
- Controlled Storage Conditions: Store products in environments that maintain specified temperature, humidity, and cleanliness standards.
- Transport Monitoring: Use validated shipping methods to ensure products remain stable during transit.
- Compliance Tracking: Regularly monitor distribution channels for compliance with GMP standards, including handling practices and storage conditions.
By addressing each of these stages meticulously, manufacturers not only meet GMP requirements but also build trust with regulators and consumers, ensuring that every product delivered is safe, effective, and of the highest quality.
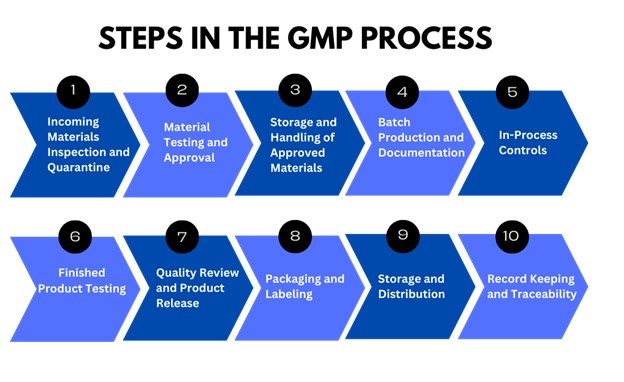
Here’s a step-by-step overview of a typical GMP process:
A typical GMP process involves several critical steps to ensure that products meet the highest standards of quality, safety, and compliance. It begins with the inspection and testing of incoming materials, followed by proper storage and handling to maintain their integrity. Each stage, from batch production and in-process controls to final testing, packaging, and distribution, is carefully monitored and documented. These steps work together to guarantee consistent, reliable products that align with regulatory requirements and protect consumer trust.
Best Practices for GMP Compliance
Implementing GMP effectively requires a proactive approach and a commitment to continuous improvement. Here are five best practices to ensure compliance and foster a culture of quality:
1. Build a Quality-First Culture
- Prioritize GMP Across the Organization: Quality should be a shared responsibility, ingrained in every department and process.
- Encourage Openness: Create an environment where employees feel empowered to report issues or suggest improvements without fear of reprisal.
- Lead by Example: Management must actively support and champion GMP initiatives to set the tone for the organization.
2. Leverage Technology
- Digital Solutions: Use advanced software tools to streamline process tracking, document management, and equipment validation.
- Automation: Automate repetitive tasks like data entry, testing, or record-keeping to reduce errors and increase efficiency.
- Data Analytics: Implement tools to monitor trends, identify risks, and optimize processes in real time.
3. Conduct Regular and Targeted Training
- Ongoing Education: Train employees on updated GMP regulations, company-specific procedures, and their roles in compliance.
- Role-Specific Training: Tailor programs to specific responsibilities, ensuring every team member knows how to contribute to GMP success.
- Assess Competency: Use quizzes or practical assessments to confirm understanding and reinforce knowledge.
4. Stay Audit-Ready
- Mock Inspections: Simulate FDA or internal audits to identify weaknesses and address them proactively.
- Documentation Updates: Keep records organized, accurate, and accessible for quick retrieval during inspections.
- Continuous Monitoring: Regularly review and refine processes to ensure ongoing compliance with changing regulations.
5. Seek Professional Support
- Consulting Expertise: Partner with GMP specialists to develop or refine your compliance strategies.
- Tailored Solutions: Work with experts who understand your industry’s specific challenges and regulatory requirements.
- Cost Savings: Avoid costly mistakes by leveraging external guidance for audits, training, and process optimization.
By integrating these practices into your operations, you can strengthen compliance, enhance product quality, and build trust with regulators and consumers alike.
FDA and GMP Compliance: What You Need to Know
The FDA enforces GMP through inspections and regulatory actions to ensure that manufacturing facilities meet safety, quality, and efficacy standards. Understanding the FDA’s role helps manufacturers maintain compliance and avoid penalties.
Key Types of FDA Inspections
- Routine Inspections: Scheduled evaluations to assess GMP compliance.
- For-Cause Inspections: Triggered by complaints or product issues.
- Pre-Approval Inspections: Conducted before the approval of new products to verify compliance.
What Happens During an Inspection?
- Documentation Review: Inspectors check SOPs, CAPAs, and other quality records for accuracy and completeness.
- Facility Walkthroughs: Inspections of manufacturing areas to evaluate cleanliness, equipment, and adherence to standards.
- Employee Interviews: Staff may be questioned to ensure they understand GMP protocols and responsibilities.
Consequences of Non-Compliance
Failing to meet GMP standards can result in:
- Form 483 Observations: Notices of potential violations requiring corrective action.
- Warning Letters: Formal demands for compliance with potential legal consequences.
- Seizures, Injunctions, or Fines: Severe penalties for persistent or egregious violations.
The Value of GMP
Good Manufacturing Practices (GMP) are more than regulatory mandates—they are the foundation of trust, quality, and operational excellence. By adhering to GMP, companies demonstrate a commitment to producing safe, effective, and high-quality products, building confidence with regulators and consumers alike. Prioritizing GMP not only ensures compliance but also enhances brand reputation, minimizes risks, and positions businesses for sustained success in competitive, regulated markets.
Need Expert Support?
Navigating GMP compliance can be complex, but you don’t have to do it alone. Our team of experienced consultants specializes in creating and executing customized compliance strategies tailored to your business needs.
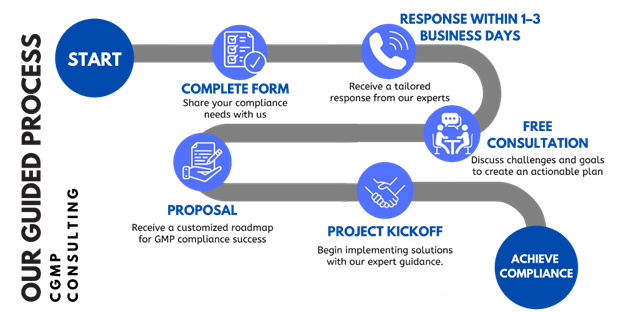
How We Guide You Through GMP Compliance
Our proven process ensures a smooth journey toward GMP compliance, with clear steps tailored to your unique needs.
Ready to get started? Complete the form today, and let’s take the first step toward GMP compliance!
Resource Guide: Current FDA CFRs
Below is a list of key FDA CFRs related to GMP compliance. Each entry includes the regulation title, a brief description, and a link to the official resource for more details:
| CFR Title | Description | Link |
|---|---|---|
| 21 CFR Part 4 | Current Good Manufacturing Practice Requirements for Combination Products | View Regulation |
| 21 CFR Part 110 | Current Good Manufacturing Practice in Manufacturing, Packing, or Holding Human Food | View Regulation |
| 21 CFR Part 111 | Current Good Manufacturing Practice for Dietary Supplements | View Regulation |
| 21 CFR Part 210 | Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs | View Regulation |
| 21 CFR Part 211 | Current Good Manufacturing Practice For Finished Pharmaceuticals | View Regulation |
| 21 CFR Part 212 | Current Good Manufacturing Practice for Positron Emission Tomography Drugs | View Regulation |
| 21 CFR Part 606 | Current Good Manufacturing Practice For Blood and Blood Components | View Regulation |
| 21 CFR Part 820 | Quality System Regulation for Medical Devices | View Regulation |
Each of these regulations addresses specific aspects of GMP compliance across various industries. By reviewing these resources, you can better understand and adhere to the regulatory requirements for your operations.
Frequently Asked Questions
GMP refers to foundational guidelines that outline best practices for manufacturing and quality control. cGMP, or “current” Good Manufacturing Practices, emphasizes the importance of using up-to-date systems, technologies, and methods to ensure compliance with evolving standards.
The FDA’s inspection frequency depends on factors such as the type of facility, the risk profile of the products, and the company’s compliance history. High-risk facilities or those with previous violations may face more frequent inspections.
Yes, small businesses can comply with GMP, even with limited resources. Tailored GMP programs and consulting support can help smaller operations meet compliance standards effectively without exceeding their budgets.
The “c” in cGMP stands for “current,” reflecting the need for companies to stay updated with the latest technologies, methods, and regulatory changes. The lowercase “c” emphasizes the importance of continuous improvement and adopting modern practices.
Non-compliance with GMP can lead to serious consequences, including:
- FDA-issued Form 483 observations or Warning Letters.
- Product recalls, fines, or legal action.
- Damage to brand reputation and loss of consumer trust.
Maintaining compliance is crucial to avoid these risks and protect both the business and its customers.
“This post was developed with input from our GMP experts and crafted by our SEO and blogging specialist, Tayler Awad, to enhance accessibility and online reach.”