Creating a Periodic Validation Review (PVR) Schedule

Understanding the Purpose of a PVR Schedule
A Periodic Validation Review (PVR) is a critical component in maintaining the integrity and compliance of qualified equipment and systems within regulated industries, such as pharmaceuticals, biotechnology, and medical devices. The primary purpose of a PVR schedule is to ensure that once equipment or systems have been qualified and commissioned, they continue to operate in a validated state. This means they consistently meet their intended critical requirements, adhere to current industry standards and regulations, and remain under proper control throughout their entire lifecycle.
Regular PVRs help identify any deviations, changes, or potential risks that could impact product quality, safety, or regulatory compliance. This proactive approach supports continuous improvement and risk management, ultimately safeguarding product integrity and patient safety.
Steps to Develop a PVR Schedule
Adding Qualified Equipment to the PVR Schedule
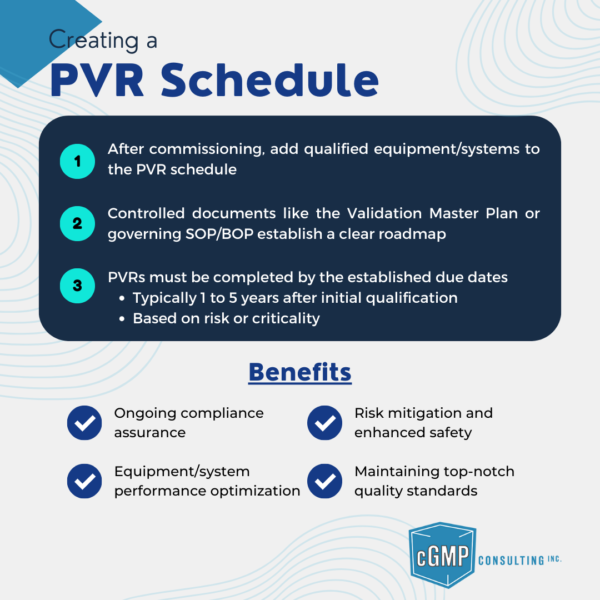
Once the equipment or system is qualified and commissioned, it is added to the PVR schedule in a controlled document, such as a Validation Master Plan or a governing SOP/BOP.
Setting Due Dates for PVR Completion
The PVR must be completed by its due date, which is typically set at either 1 year or 5 years after the initial qualification, depending on the risk level or criticality of the equipment.
cGMP Consulting can help with your PVR or other compliance needs. Contact us today!





