
Your Complete Guide to Audit Readiness: The 5 Step Process
In regulated manufacturing industries, being audit-ready is not just a good practice; it’s a necessity. The FDA can inspect facilities without notice, so it’s essential to always have your organization ready. Up front preparation can make all the difference.
At cGMP Consulting, we understand the complexities and nuances of maintaining compliance and preparing for inspections. That’s why we offer specialized Audit Readiness Training to equip your team with the skills and knowledge necessary to excel in any audit scenario.
Audit readiness is essential for several reasons:
- Regulatory Compliance: Maintaining compliance is crucial because it ensures product safety and efficacy, protects public health, and helps companies avoid regulatory actions and other preventable compliance issues.
- Operational Efficiency: A well-prepared team can streamline processes, reduce downtime, and enhance overall productivity during audits, making the audit experience smoother, less stressful, and less disruptive.
- Reputation Management: Being audit-ready reflects your company’s commitment to quality and regulatory adherence, enhancing your reputation with stakeholders, clients, and consumers.
Why Audit Readiness is Relevant
With increased FDA budgets for 2024 and 2025, the agency is ramping up enforcement and inspections. As the FDA focuses more on compliance, your customers will also be more vigilant regarding regulatory adherence.
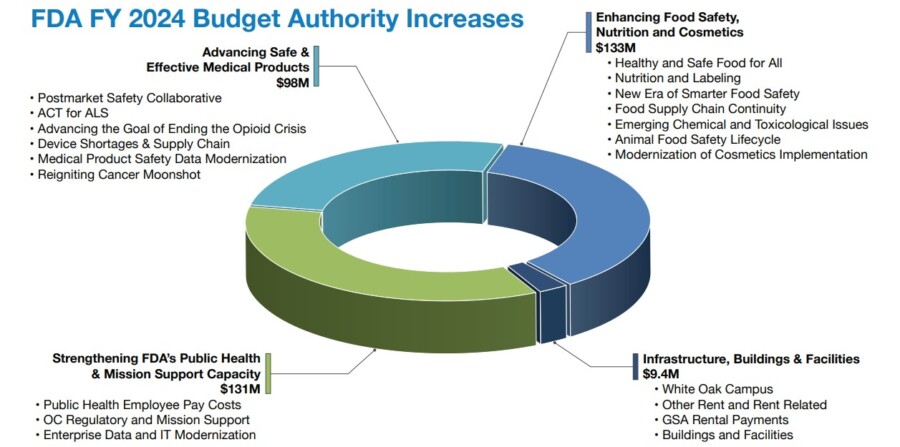
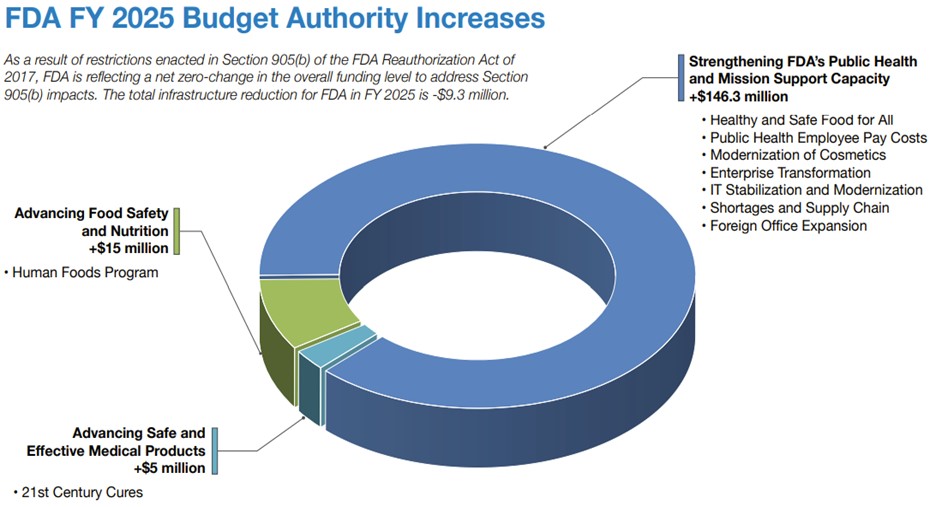
Here you will see the FDA budget allocations for 2024 and 2025.
Comprehensive Process: Audit Readiness Training
Our Audit Readiness Training is designed to cover all aspects of regulatory inspections and even internal audits, specific to your industry. Here’s what you can expect:
1. Understanding Regulatory Requirements
Our training begins with a deep dive into the latest regulations relevant to your industry, including:
- Current Good Manufacturing Practices (cGMP)
- FDA regulations guidelines
- Any additional standards, international or otherwise, and their implications
2. Audit Preparation Strategies
Your team will learn essential preparation strategies, focusing on:
- Facility and process preparation
- Confirm your site is tidy, well organized by conducting a walk-through. Clutter will only frustrate the auditor and show them that your products run the risk of contamination and/or mix-ups.
- Identify adequate space for your auditors and your internal audit team to work comfortably.
- Determine the roles and responsibilities of your internal audit team.
- Determine the flow of information between the auditors and your team.
- Establish a protocol for the arrival of regulatory auditors.
- Documentation and record-keeping best practices
- It’s crucial to have all official documents available for the auditor, having to search for something while being inspected will diminish credibility.
- Ensure you have all documents up to date including such things as organizational charts, facility layout, Quality Manual, SOPs, Batch Records, and all training documentation.
- Have a system to track all the documents that are requested during the audit
- Preparing your staff
- Make sure your entire staff is aware and what expectations you have of them.
- Prepare for what could go wrong and how to handle.
- Make sure someone is always available and dedicated to facilitating the audit.
3. The Audit: How to Ensure a Smooth Process
- Audits typically start with a facility tour; we will share best practices.
- Identify key SMEs to escort the auditor(s) and have a plan in place in case someone identified is on vacation.
- Restrict conversations to area being toured.
- Have relevant SOPs ready for each process or work performed in each area.
4. Effective Communication Skills
Learn how to communicate effectively with auditors, including:
- Choosing the right person from your organization to present is critical for a successful audit, someone who can present information clearly and confidently.
- Be courteous and professional.
- Respond to auditor questions and requests without adding additional information.
- Managing stressful situations during audits and avoiding arguments or defensive behavior.
5. Post-Audit Activities
Understand the importance of post-audit activities such as:
- What should be in a daily wrap up meeting and/or close out meeting
- Getting a clear understanding of the auditor’s findings or concerns raised
- Corrective and preventive action (CAPA) planning
- Implementing audit recommendations
- Continuous improvement strategies
- Audit response documentation
Benefits of Our Training Programs
- Expert Consultants: Our trainers are industry veterans with extensive regulatory and audit experience that have been key players in numerous audits in various industries.
- Customized Training: Programs are tailored to meet the specific needs of your organization.
- Practical Insights: Gain valuable insights from real-world case studies and best practices from experts that can recommend practical solutions.
- Comprehensive Support: Access to all our compliance services through knowledgeable consultants for ongoing support and advice.
- Hands on Experience: Mock audits are vital; they provide:
- Real-world scenarios to practice responses
- Identification of potential non-compliance issues
- Practice with “typical questions”
- Constructive feedback to improve readiness
Achieve Audit Excellence with cGMP Consulting
Don’t wait until an audit notice to start preparing. With cGMP Consulting’s Audit Readiness Training, you can ensure your team is always ready to meet regulatory expectations with confidence.
Reach out today to schedule a call to discuss your unique business needs.





