Technical Writing: Deviations
Enhancing Compliance Through Effective Deviation Management
Background:
Technical writing is paramount to FDA-regulated industries, where clear and accurate reporting is vital to prevent product loss, financial repercussions, or harm to patients. The process of conveying complex information in a manner that is easily understandable to a third-party is essential to successful technical writing. Deviations are an aspect of the industry where good technical writing is essential.
Understanding Deviations
A deviation, often referred to as an event, encompasses anything unplanned or unexpected from an approved procedure, guideline, or specification. Deviations can occur at any stage of product preparation, manufacturing, packaging, sampling, or testing. An example of a deviation is a product filter integrity test failure after said filter was used during a production batch. Deviations should be initiated and documented within 24 hours of discovery in the Quality Management System (QMS) database and any product that is affected must be placed on hold (keep from being released to public).
The Deviation Process Flow
The deviation process flow has 5 main phases:
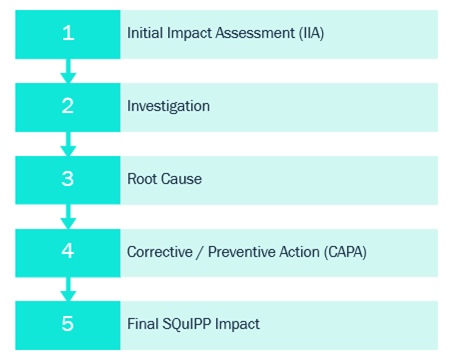
1. Initial Impact Assessment:
The Initial Impact Assessment (IIA) is used to identify the scope and severity of the event.
The impact assessment should provide a clear description of the event. The impact is identified against SQuIPP; in other words, how does this event impact Safety, Quality, Identity, Purity, and Potency of the product?
Based on the impact assessment, a Risk Probability Number (RPN) is assigned based on the probability, detectability, and severity of the event. Typically, the IIA needs to be completed within a specific timeframe after the event occurs (i.e. three days). This is outlined in company-specific procedures.
2. Investigation:
An investigation should be conducted to outline the details surrounding the event. The investigation may include but is not limited to:
- Interviews: It is important to discuss the event with multiple people that were involved within the situation. This is key to ensure that all the evidence leads in the same direction.
- Training Review: Verify the training records. Did the personnel involved have sufficient training to perform the tasks that were completed?
- Data Review: Review trends, audit trails, alarm records, etc. to determine if abnormalities occurred around the time of the event.
- Batch Record Review: Ensure all the required tasks were completed as per the procedure and documented in the batch record.
The investigation should be written so that an outside third party (i.e. auditor) can understand the process, what deviated, and can correctly interpret the data behind the event.
3. Root Cause
A systemic approach should be used to determine the root cause based on the findings of the investigation.
Multiple root cause analysis tools are available to help determine the root cause including:
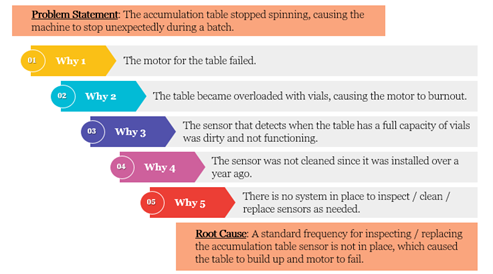
- Five-Whys-Technique: This tool is simple in execution – simply ask “Why?” five times to determine the root cause. This technique can help a team solve simple problems relatively quickly. An example of the 5-Whys can be seen here:
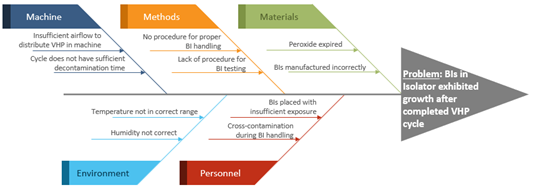
- Fishbone: A fishbone, or cause-and-effect diagram, is a method to identify many possible causes for a problem. The fishbone tool should be used after a detailed problem statement has been determined and is often used in a brainstorming session. Typically, the potential problems are sorted into different categories in the diagram. An example of a fishbone can be seen here:
4. Corrective and Preventive Action (CAPA):
- A corrective action is an action to eliminate the cause(s) of an event / deviation with the intention to prevent recurrence.
- A preventive action is an action to eliminate the cause(s) of a potential event / deviation to avoid occurrence in other related processes, systems, or facilities.
CAPAs are required for most events / deviations unless there is justification for not implementing one. Not all CAPAs are created equal and can have varying degrees of effectiveness in eliminating reoccurrence, ranging from strong actions to week actions.
- A strong action is removing the hazard 100% through engineering controls.
- A weak action is influencing human behavior through trainings, typically awareness trainings.
As part of the CAPA implementation, typically an effectiveness check will be assigned to ensure that there were no recurrences of the event and that the CAPA was “effective”. Timeframes for effectiveness checks can vary greatly, typically ranging from 3 months to a year.
5. Final SQuIPP impact:
Following the investigation, root cause analysis, and CAPA, a final impact will be assigned to the deviation (critical, major, or minor).
This allows the product to be released from its respective quality hold and delivered to patients depending on the impact.
Common Issues in Deviation / Event Investigations
- Incomplete Investigations: During investigations, it is common to stop at the first and most obvious conclusion without looking at all the information or supporting data. Additionally, in some instances, after completing the initial investigation without a clear root cause, a team may conclude that there is no definitive root cause without diving deeper into the data and troubleshooting.
- Assumptions: False assumptions are made during deviation investigations as well, where the investigator thinks that they know the process and does not ask questions of other parties that are involved in the event. False assumptions can lead to incorrect conclusions, and a recurrence of the same issue.
- Narrow in Scope: An investigator may not look at all potential causal factors within the event, or the investigation was not extended to other potentially impacted processes or products. An example of this is investigating a low water pressure alarm occurrence on a filling line vial washer without looking at the data and alarms that are occurring on the other filling line vial washers. This can result in a CAPA that is narrow in scope and does not fully resolve the problem.
- Inadequate Product Impact Assessment: The risk may not be assessed for other products or lots that are impacted by the deviation, resulting in risk to patients.
Conclusion
At cGMP Consulting, our skilled technical writers excel not only in deviation investigations but also in protocols, standard operating procedures, and other compliance documentation. Strong technical writing is necessary not just to ensure smooth business operations, but also to maintain proper documentation and to ensure patient or customer safety.
For expert guidance on deviation investigations and compliance documentation, contact us today.